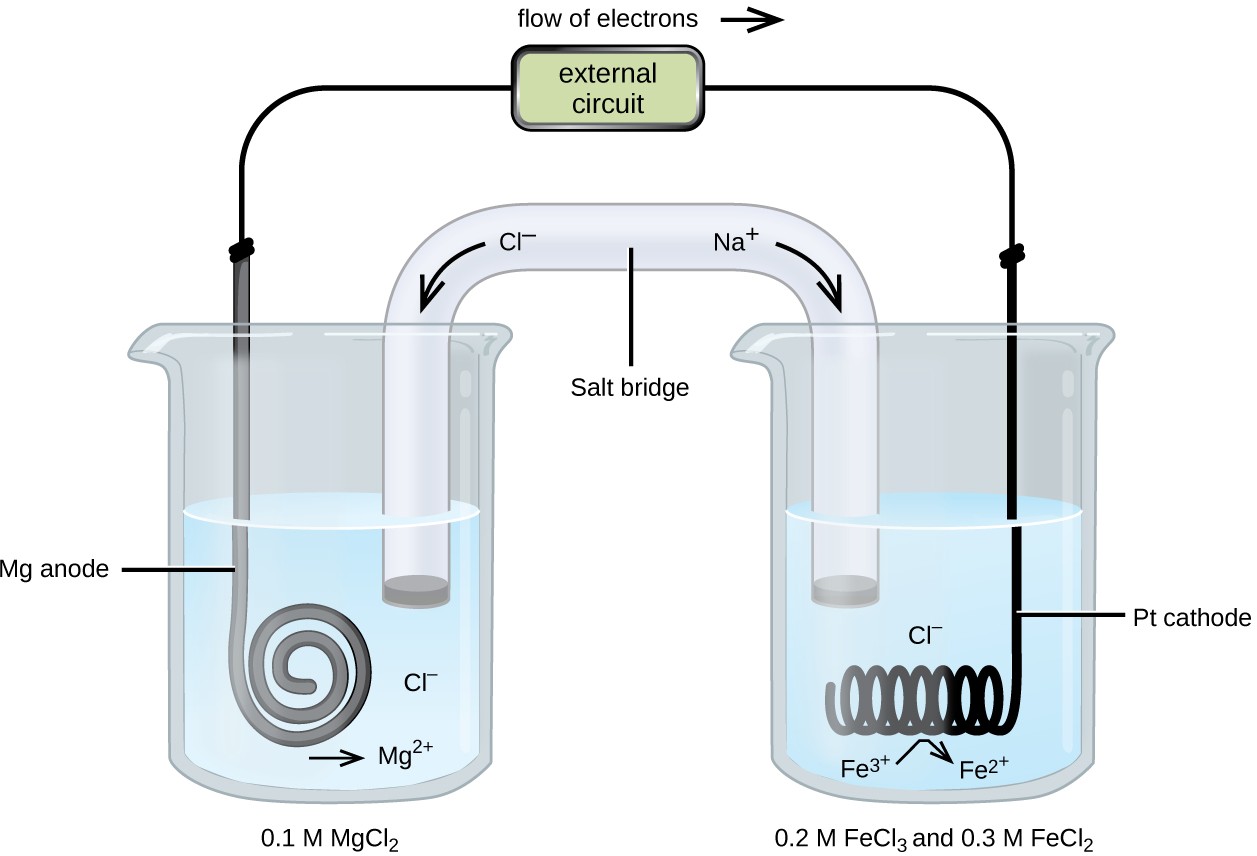
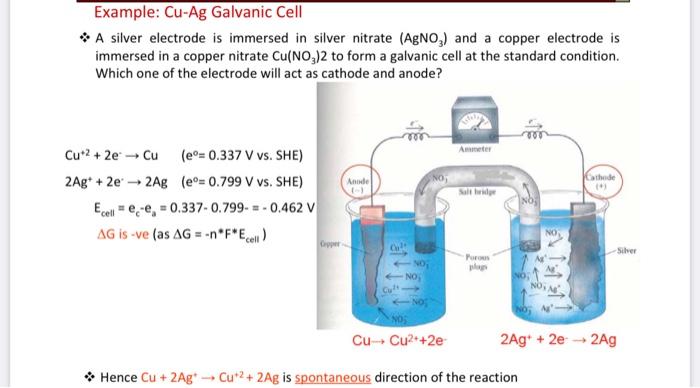
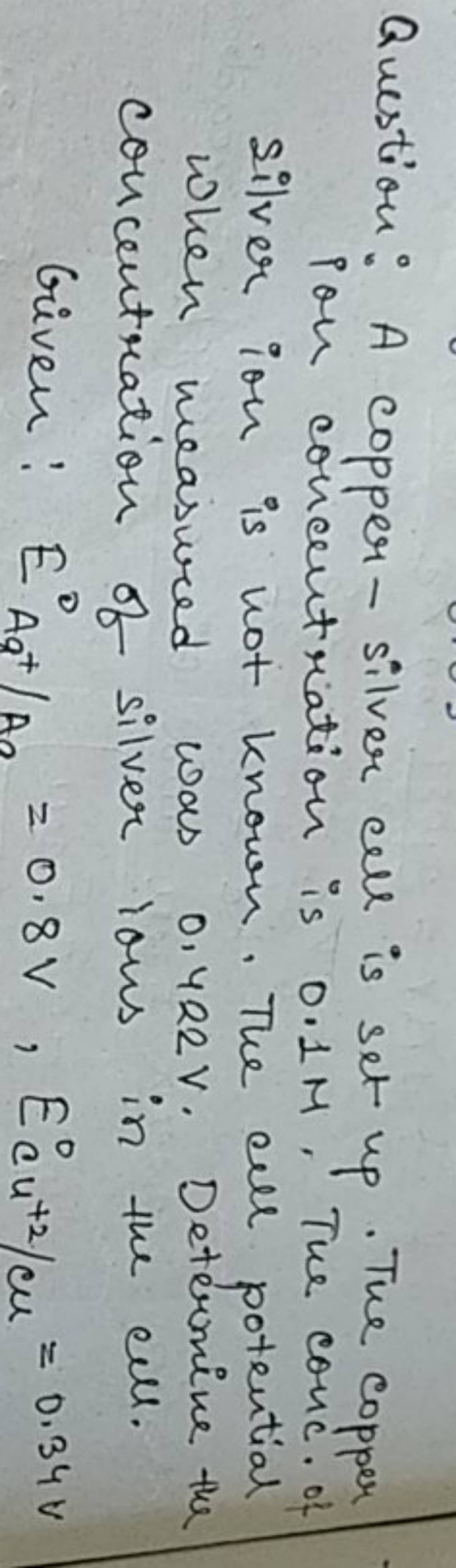13.A copper-silver cell is up. The copperion concentration is 0.10M. The concentration of Silver ions is not known. The cell potential was found to be 0.422V.Determine the concentration Silver ion in
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. - Sarthaks eConnect | Largest Online Education Community

A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver - Brainly.in

III. Cell Potentials Under Standard Conditions B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that includes all the components (

Solve this: 8 A Copper-Silver cell is set up The copper ion - Chemistry - Electrochemistry - 12485096 | Meritnation.com
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. - Sarthaks eConnect | Largest Online Education Community
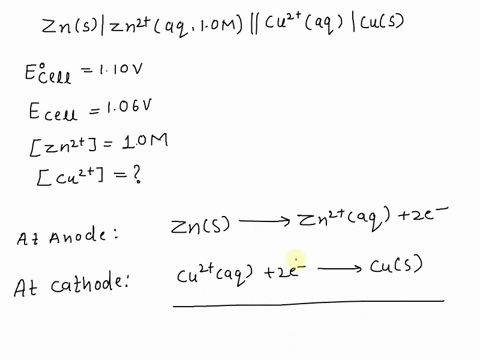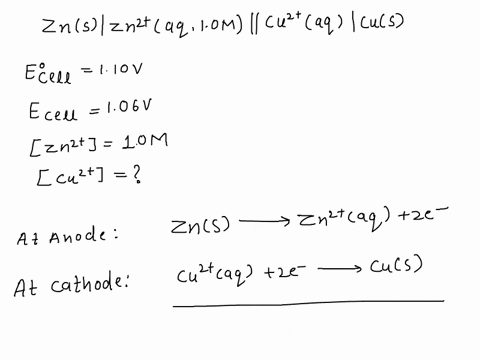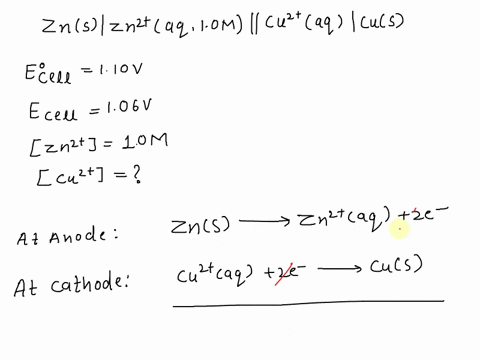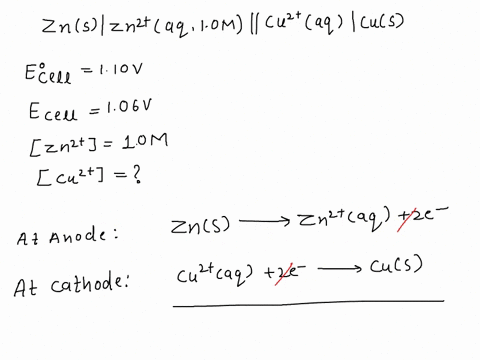
SOLVED: A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential is measured as 0.422 V.

7. of A copper silver cell is setup. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measures 0.422V. Determine the

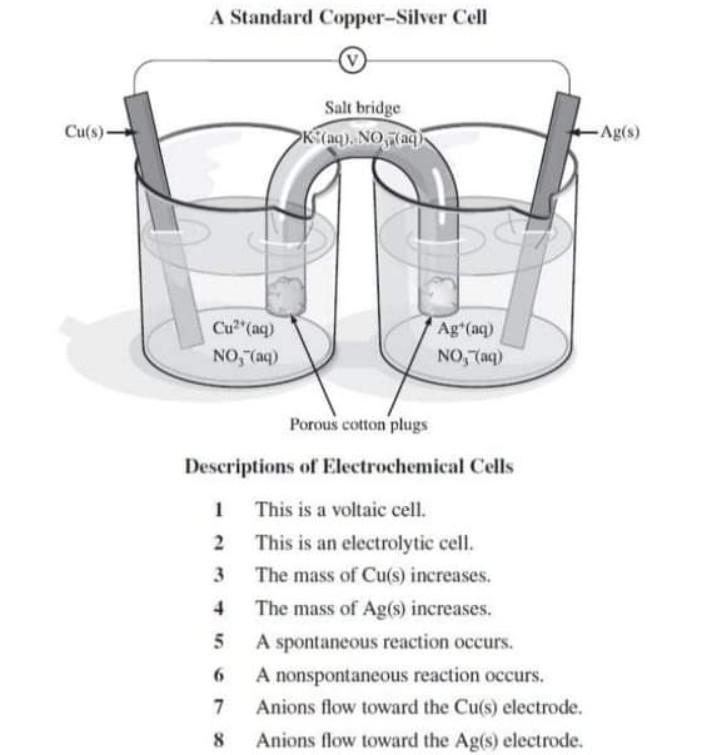
![Assamese] A copper silver cell is set up. The copper ion concentrati Assamese] A copper silver cell is set up. The copper ion concentrati](https://static.doubtnut.com/ss/web-overlay-thumb/8484396.webp)